Background
Morpholino antisense oligos (PMOs), a synthetic DNA analog, block sites on RNA and can make targeted changes in gene expression. PMOs have been widely used in developmental biology to bind RNA transcripts in embryos of model organisms. In embryos, PMOs can be conveniently delivered by microinjection into the cytosol of early zygotes. This has demonstrated the power and flexibility of the PMO approach when delivery is not an issue. In adult animals, the delivery challenge has inhibited the widespread application of PMOs. PMOs have been slow to enter the pharmaceutical field as pharmacologically active compounds. This is largely due to their poor uptake across the plasma membrane of cells.
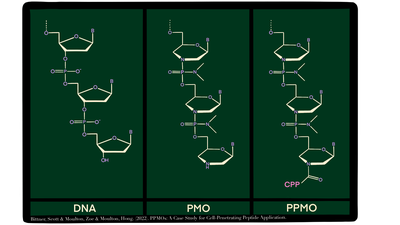
There are several FDA-approved PMO drugs, but so far these are limited to treatment of Duchenne muscular dystrophy (DMD). In DMD regenerating muscle fuses with satellite cells that have taken up PMOs, delivering small doses of the oligos to the site where they are needed for therapeutic effect. To apply PMOs with better efficacy for DMD and more broadly in human diseases, good techniques for enhancing their delivery in vivo are needed.
There is an additional need for the effective delivery of PMOs into bacterial cells without toxicity to human cells, allowing therapeutic targeting of PMOs to bacterial diseases. Other applications involve targeting PMOs for uptake into different animals, such as our current work on enhanced PMO delivery into corals to study the mechanisms of coral disease.